EBS recently assisted a paper mill with odor issues associated with their aerated stabilization basin (ASB) wastewater treatment system. The formation of reduced sulfur compounds (hydrogen sulfide, methyl mercaptan, dimethyl sulfide) occurred in a large unaerated holding pond that followed the aerated basin, leading to odor complaints from the community. The odor from reduced sulfur compounds (RSCs) can be a difficult issue to deal with, as other effluent parameters (BOD, TSS) can be well within permit limitations, but these odor-causing compounds can still be produced.
As explained in the simplified Electron Tower in Figure 1, heterotrophic bacteria preferentially utilize oxygen for cellular respiration, which produces odorless CO2 as a byproduct. If oxygen isn’t present, they prefer nitrate if it is available, which produces odorless N2 gas. Lastly, if neither oxygen nor nitrate is present, the bacteria utilize sulfate, which produces odorous hydrogen sulfide and other RSCs. A large quantity of sulfate is typically present in pulp and paper mill effluents.
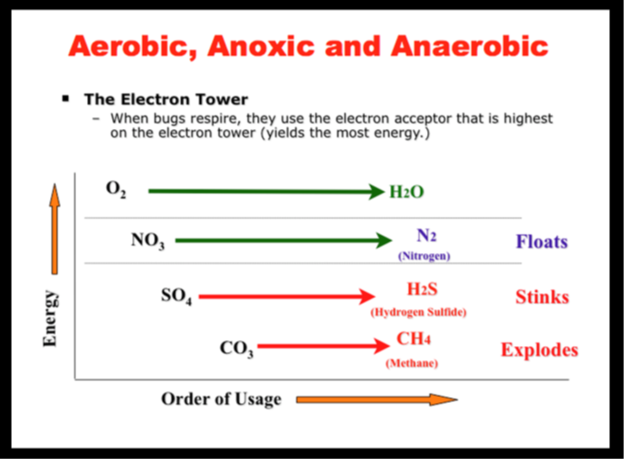
Figure 1
What causes RCS formation in my treatment system?
To understand mitigation strategies, it’s important to understand how these odors are generated in large settling basins after aeration. Typically, most of the RSCs coming from the process are oxidized in the aeration basin, as they quickly react with the oxygen supplied by the aerators. However, these compounds can form again in the treatment system if the following conditions occur:
-
- moderate/high concentration of BOD is still present
- dissolved oxygen and nitrate concentrations are negligible
- sulfate concentration is high
How can I prevent RSC formation from occurring?
To prevent the formation of RSCs in the Final Holding Pond, EBS worked with the mill to:
-
- Deploy strategies to minimize soluble BOD in the ASB Effluent.
- Utilize supplemental nitrate (EBS CalNit) to ensure nitrate was utilized instead of sulfate if BOD carryover occurred into the final holding pond.
- Utilized additives (ferric chloride) that would precipitate RSCs if formed in the holding pond.
Innovative solutions for RSC formation:
A supplemental nutrient program was initiated to improve BOD removal efficiency in the ASB, and mill personnel did an excellent job of monitoring and limiting organic loading into the treatment system. RSC formation in the holding pond was significantly reduced in a short period of time.
As a result of this and other similar opportunities, EBS is introducing FerroxTM, a ferric nitrate solution. This unique product provides the powerful combination of nitrate as a terminal electron acceptor to prevent H2S formation and ferric ions to precipitate any existing aqueous sulfide molecules.
To learn more about this creating option, If you’re dealing with odor issues, give us a call today!

